
Representative PI hydrolysis data:
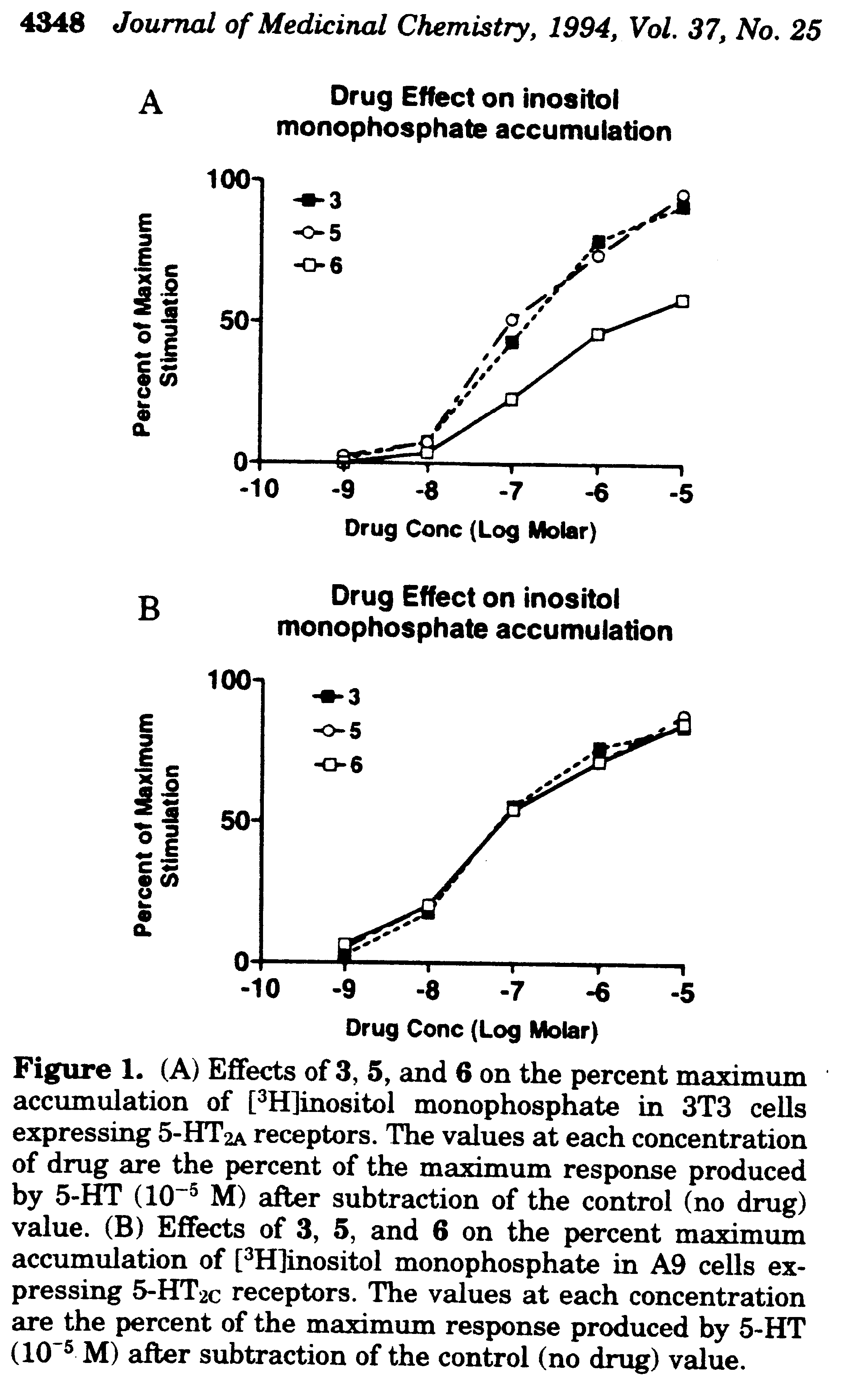
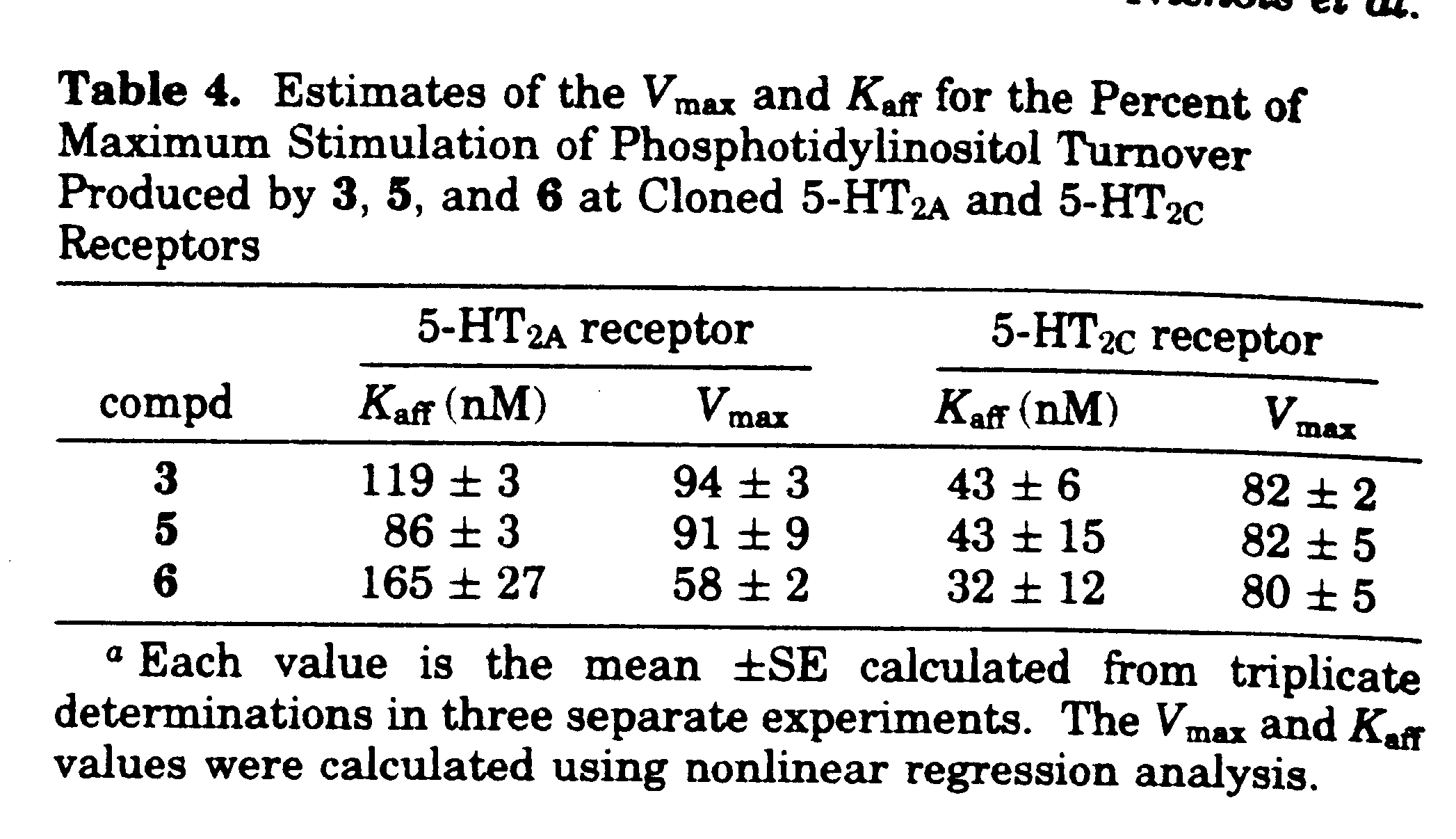
Representative Kact and %Vmax data for PI hydrolysis protocol:
Template for Kact and %Vmax determinations in 24-well plate format:
| BLANK | BLANK | BLANK | 10 mM STD #1 |
10 mM STD #1 |
10 mM STD #1 |
| 10 mM STD #2 |
10 mM STD #2 |
10 mM STD #2 |
10 mM UNK #1 |
10 mM UNK #1 |
10 mM UNK #1 |
| 10 mM UNK #2 |
10 mM UNK #2 |
10 mM UNK #2 |
10 mM UNK #3 |
10 mM UNK #3 |
10 mM UNK #3 |
| 10 mM UNK #4 |
10 mM UNK #4 |
10 mM UNK #4 |
10 mM UNK #5 |
10 mM UNK #5 |
10 mM UNK #5 |
|
For calculation of Kact and Vmax values, SigmaPlot V4.0 is used to fit the raw
data to the following equation: V= Vmax * X + NSA Kact + X | |
| Where V=Activity at each concentration of X (compound of interest);
X=concentration of compound of interest; Vmax=parameter to be estimated;
Kact=parameter to be estimated; NSA=non-specific activity at baseline. We have
used the procedure extensively to calculate Vmax and Kact values. | |
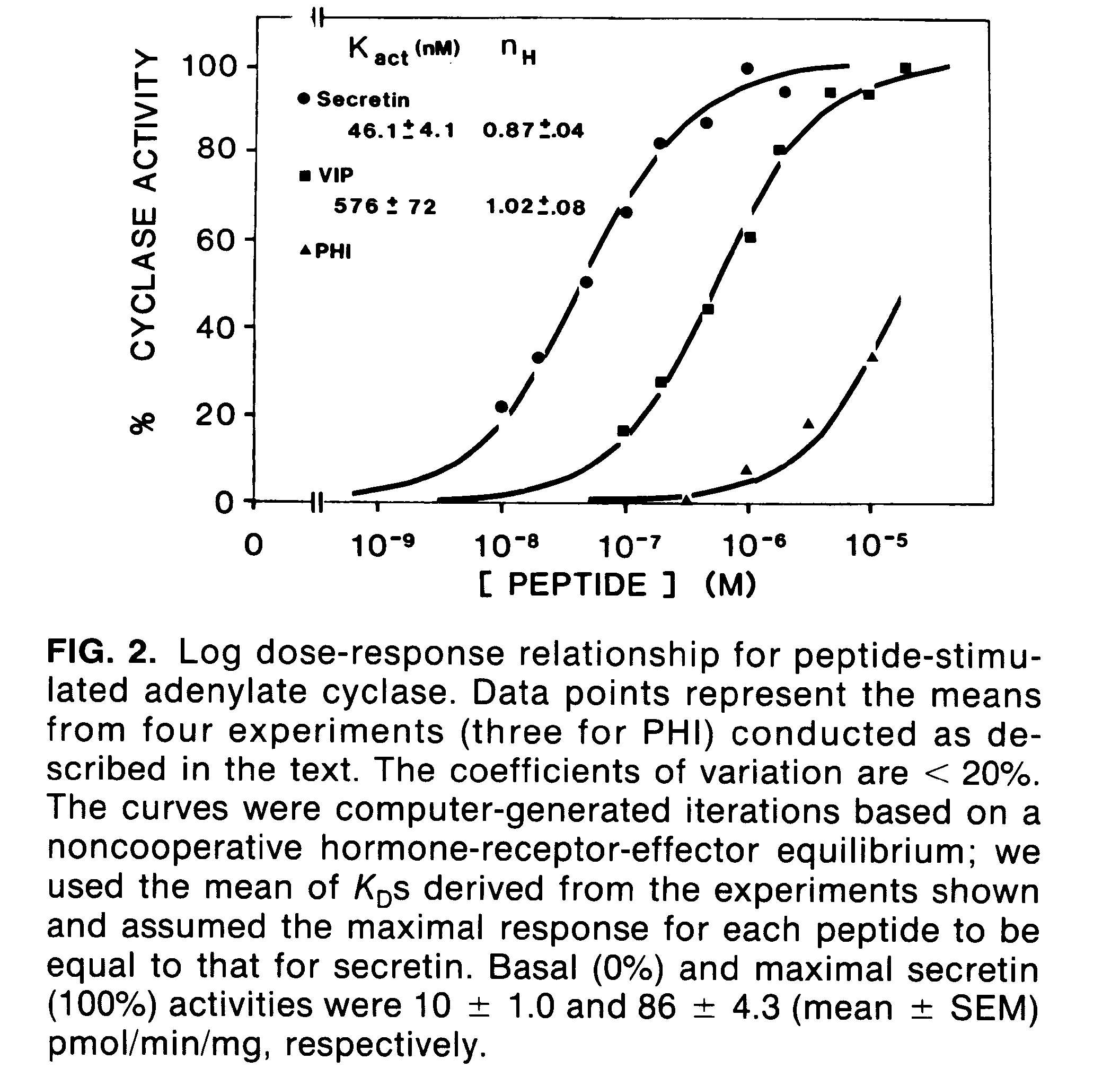
Representative cAMP stimulation data with Kact values and Hill Coefficients calculated:
Template for Kact and %Vmax determinations in 24-well plate format:
| BLANK | BLANK | BLANK | 10 mM STD #1 |
10 mM STD #1 |
10 mM STD #1 |
| 10 mM STD #2 |
10 mM STD #2 |
10 mM STD #2 |
10 mM UNK #1 |
10 mM UNK #1 |
10 mM UNK #1 |
| 10 mM UNK #2 |
10 mM UNK #2 |
10 mM UNK #2 |
10 mM UNK #3 |
10 mM UNK #3 |
10 mM UNK #3 |
| 10 mM UNK #4 |
10 mM UNK #4 |
10 mM UNK #4 |
10 mM UNK #5 |
10 mM UNK #5 |
10 mM UNK #5 |
| For calculation of Kact and Vmax
values, SigmaPlot V4.0 is used to fit the raw data to the following equation: V= Vmax * X + NSA Kact + X |
|
|
Where V=Activity at each
concentration of X (compound of interest); X=concentration of compound of interest;
Vmax=parameter to be estimated; Kact=parameter to be estimated; NSA=non-specific activity
at baseline. We have used the procedure extensively to calculate Vmax and Kact values.
|
|
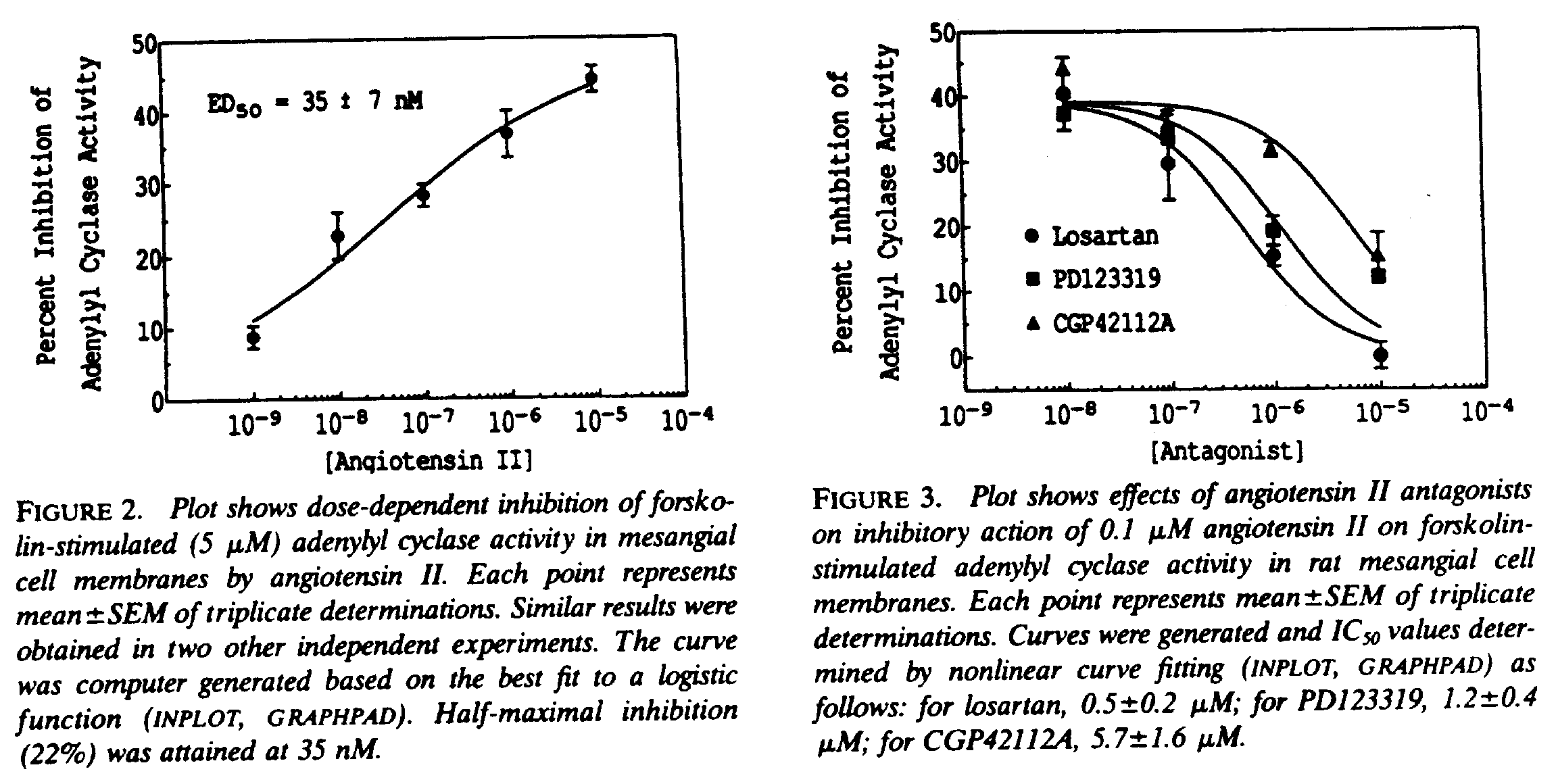
Inhibition of forskolin-stimulated adenylate cyclase will be performed as previously detailed by us . In brief, cells will be grown in 24-well plates as described above, switched to serum-free medium for 4 hr and then stimulated with forskolin in the presence and absence of test agents. cAMP accumulation will be measured with a commercially available kit. The % inhibition of forskolin-stimulated adenylate cyclase activity will then be calculated and the data fit to the Kact and Vmax equation.
Representative cAMP inhibition data:
Template for Kact and %Vmax determinations in 24-well plate format:
| BLANK | BLANK | BLANK | 10 mM STD #1 |
10 mM STD #1 |
10 mM STD #1 |
| 10 mM STD #2 |
10 mM STD #2 |
10 mM STD #2 |
10 mM UNK #1 |
10 mM UNK #1 |
10 mM UNK #1 |
| 10 mM UNK #2 |
10 mM UNK #2 |
10 mM UNK #2 |
10 mM UNK #3 |
10 mM UNK #3 |
10 mM UNK #3 |
| 10 mM UNK #4 |
10 mM UNK #4 |
10 mM UNK #4 |
Forskolin | Forskolin | Forskolin |
| For calculation of Kact and Vmax
values, SigmaPlot V4.0 is used to fit the raw data to the following equation: V= Vmax * X + NSA Kact + X |
|
|
Where V=Activity at each
concentration of X (compound of interest); X=concentration of compound of interest;
Vmax=parameter to be estimated; Kact=parameter to be estimated; NSA=non-specific activity
at baseline. We have used the procedure extensively to calculate Vmax and Kact values.
|
|
Template for Kact and %Vmax determinations in 24-well plate format:
| BLANK | BLANK | BLANK | 10 mM STD #1 |
10 mM STD #1 |
10 mM STD #1 |
| 10 mM STD #2 |
10 mM STD #2 |
10 mM STD #2 |
10 mM UNK #1 |
10 mM UNK #1 |
10 mM UNK #1 |
| 10 mM UNK #2 |
10 mM UNK #2 |
10 mM UNK #2 |
10 mM UNK #3 |
10 mM UNK #3 |
10 mM UNK #3 |
| 10 mM UNK #4 |
10 mM UNK #4 |
10 mM UNK #4 |
10 mM UNK #5 |
10 mM UNK #5 |
10 mM UNK #5 |
--Cells are seeded at 1x105 cells/ml into 24-well plates and grown overnight in medium containing dialyzed serum. The next day, cells are switched to a modified KRB buffer (see above) and pre-incubated for 20 min at 37 C. Cells are then incubated with 1 uM (final concentration) 3H-5HT, DA or NE for 4 min. The reaction is then terminated by aspiration and the cells washed three times with ice-cold incubation buffer. Cells are then dissolved with 500 ul of 0.5 N NaOH with 350 ul added to AQUASURE and radioactivity measured after chemilumiscence has decayed (generally overnight).
--Representative Ki values for inhibition of 5-HT uptake found in Nakaki et al, 1995 and Palvimakki et al, 1996.