- Introductory Statement of Purpose and General Plan
- Study Protocols: PET Imaging of CB1 Receptors Using [18F]FMPEP-d2
- Chemistry, Manufacture and Controls
- Pharmacology and Toxicology
- CB1 Human Pharmacology and Toxicology
- In Vitro Receptor Binding
- Extended Acute Toxicity Study in Rat
- Two-week Repeat Dose Toxicity Study in Rats
- S. typhimurium-E. coli/Mammalian-Microsome Reverse Mutation Assay
- In Vitro Microwell Micronucleus Assay in CHO Cells
- Human Studies
- Animal Experimentation
- PET Imaging of [18F]FMPEP-d2 in Nonhuman Primates
- Pharmacology of FMPEP-d2 in Nonhuman Primates
- Estimated Receptor Occupancy
- Radiation Dosimetry Estimation from Nonhuman Primates
- Human Experience
- Environmental Assessment
- Case Report Form
- References
- Appendices
- CV of Investigator
- Study Protocol
- IRB Protocol Approval
- Case Report Form
- [18F]FMPEP-d2: Dosimetry Report from Nonhuman Primates
- Paper on PET Imaging of [11C]MePPEP in Nonhuman Primates
- Brain Imaging Report from [11C]MePPEP in Humans
- MePPEP: Extended Acute Toxicity Study in Rats
- MePPEP: Two-week Repeat Dose Toxicity Study in Rats
- MePPEP: S. typhimurium-E. coli/Mammalian-Microsome Reverse Mutation Assay
- MePPEP: In Vitro Microwell Micronucleus Assay in CHO Cells
| CHEMISTRY, MANUFACTURING, AND CONTROLS | |
| Document No. | Section |
| 1 | Chemistry, Manufacturing, and Controls |
| 2 | Standard Operating Procedures |
| 3 | Master Batch Record |
| 4 | Quality Control Record |
| 5 | Post Release Test Record |
| 6 | Data Record for SOP #QA305 |
| 7 | Precursor and Reference Standard Acceptance Form |
| 8 | Certificates of Analyses |
| 9 | Validation Record |
- Introductory Statement of Purpose and General Plan
- Overview
- Scientific Background
- Statement of Purpose
- Study Protocols: PET Imaging Using [18F]FBR
- Précis - Brain Imaging
- Précis - Whole Body Dosimetry
- Précis - Test/Retest Brain Imaging
- Chemistry, Manufacture and Control
- Pharmacology and Toxicology
- CB1 Human Pharmacology and Toxicology CB1 receptors mediate diverse effects in humans, related in part to their widespread distribution in the body. CB1 receptors are found in several brain areas (cerebellum, basal ganglia, hippocampus, cortex, hypothalamus, and pituitary gland), and in a variety of peripheral tissues, including adipose tissue, gastro intestinal tract, adrenal glands, sympathetic ganglia, heart, lung, liver, testis, eye, and urinary bladder.
- In Vitro Receptor Binding. Eli Lilly tested MePPEP, rimonabant, and FMPEP in vitro and found that both have potent antagonist and inverse agonist properties. The results are described below:
- Extended Acute Toxicity Study in Rats The current exploratory IND application is based on the principle that animal toxicity data from closely related chemical analogues for a single target can be used to study an investigational candidate in a limited number of subjects. [18F]FMPEP-d2 is a closely related analogue to MePPEP. Stanford Research Institute (SRI) performed an extended acute toxicity study in rats. This study was included in exploratory IND #76,894. The executive summary is provided below, and the complete report is located in Appendix H. Please note that compound "C1" is MePPEP.
- Two-week Repeat Dose Toxicity Study in Rats Stanford Research Institute performed three additional toxicology studies on MePPEP since our initial exploratory IND application. The executive summary is provided below. The complete report is located in Appendix X.I. Please note that compound "2463608" is MePPEP.
- S. typhimurium-E. coli/Mammalian-Microsome Reverse Mutation Assay The executive summary is provided below. The complete report is located in Appendix X.J. Please note that compound "2463608" is MePPEP.
- In VitroMicrowell Micronucleus Assay in CHO Cells The executive summary is provided below. The complete report is located in Appendix X.K. Please note that compound "2463608" is MePPEP.
- Human Studies
- Animal Experimentation
- PET Imaging of [18F]FMPEP-d2 in Nonhuman Primates
- Pharmacology of FMPEP-d2 in Nonhuman Primates
- CLEARANCE The radioligand was quickly metabolized and represented 83 ± 12%, 53± 17%, 21 ± 7%, and 14 ± 5% of total plasma activity at 5, 10, 30, and 60 min, respectively, in all the four monkey studies. [11C]MePPEP was rapidly removed from arterial plasma, with a clearance rate of 361 ± 40 mL min-1. Plasma activity of unchanged [11C]MePPEP peaked at ~1 min and decreased to 3.1 ± 0.9% of the peak by 10 min.
- EFFECTS In PET scans, the injected mass dose of [11C]MePPEP was 0.11 ± 0.06 µg/kg. In all baseline and blocking experiments using ISPB (1.0 - 1.5 mg/kg) and rimonabant (3.0 mg/kg), the differences between pre- and post-injection vital signs were: < 17 mm Hg for systemic blood pressure, < 17/min for pulse, < 3/min for respiratory rate, and < 0.1 °C for temperature.
- Estimated Receptors Occupancy
- Radiation Dosimetry Estimation from Nonhuman Primates The complete report is located in Appendix X.E. The Effective Dose is estimated as 78 mrem/mCi. Thus, the proposed injected activity of 20 mCi would cause 1.56 rem Effective Dose.
- Human Experience
- Environmental Assesment
- Case Report Form
- References
-
Abood ME, Ditto KE, Noel MA, Showalter VM, Tao Q (1997): Isolation and expression of a mouse CB1 cannabinoid receptor gene. Comparison of binding properties with those of native CB1 receptors in mouse brain and N18TG2 neuroblastoma cells. Biochem Pharmacol 53:207-214.
-
Bramlage P, Muhlen I, Randeva H, Spanswick D, Lehnert H (2006): Cardiovascular risk man-agement by blocking the endocannabinoid system. Exp Clin Endocrinol Diabetes 114:75-81.
-
Burns HD, Van Laere K, Sanabria-Bohorquez S, Hamill TG, Bormans G, Eng WS, et al (2007): [18F]MK-9470, a positron emission tomography (PET) tracer for in vivo human PET brain imaging of the cannabinoid-1 receptor. Proc Natl Acad Sci U S A 104:9800-9805.
-
Cleland JG, Ghosh J, Freemantle N, Kaye GC, Nasir M, Clark AL, Coletta AP (2004): Clinical trials update and cumulative meta-analyses from the American College of Cardiology: WATCH, SCD-HeFT, DINAMIT, CASINO, INSPIRE, STRATUS-US, RIO-Lipids and car-diac resynchronisation therapy in heart failure. Eur J Heart Fail 6:501-508.
-
Despres JP, Golay A, Sjostrom L (2005): Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med 353:2121-2134.
-
Gelfand EV, Cannon CP (2006a): Rimonabant: a cannabinoid receptor type 1 blocker for man-agement of multiple cardiometabolic risk factors. J Am Coll Cardiol 47:1919-1926.
-
Gelfand EV, Cannon CP (2006b): Rimonabant: a selective blocker of the cannabinoid CB1 re-ceptors for the management of obesity, smoking cessation and cardiometabolic risk factors. Expert Opin Investig Drugs 15:307-315.
-
Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J (2006): Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. Jama 295:761-775.
-
Schou M, Halldin C, Sovago J, Pike VW, Hall H, Gulyas B, et al (2004): PET evaluation of novel radiofluorinated reboxetine analogs as norepinephrine transporter probes in the monkey brain. Synapse 53:57-67.
-
Sprague DR, Chin FT, Liow JS, Fujita M, Burns HD, Hargreaves R, et al (2007): Human biodis-tribution and radiation dosimetry of the tachykinin NK1 antagonist radioligand [18F]SPA-RQ: comparison of thin-slice, bisected, and 2-dimensional planar image analysis. J Nucl Med 48:100-107.
-
Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rossner S (2005): Effects of the cannabi-noid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet 365:1389-1397.
-
Yasuno F, Brown AK, Zoghbi SS, Krushinski JH, Chernet E, Tauscher J, et al (In press): The PET radioligand [11C]MePPEP binds reversibly and with high specific signal to cannabinoid CB1 receptors in nonhuman primate brain. Neuropsychopharmacology.
- Appendices
- Investigator data: CV
- Study Protocol
- IRB Protocol Approval
The protocols are under review by the CNS (central nervous system) IRB of NIH. This study will not proceed until it is approved by both the FDA and the NIH IRB. - Case Report Form
- [18F]FMPEP-d2: Dosimetry Report from Nonhuman Primates
- Paper on PET Imaging of [11C]MePPEP in Nonhuman Primates
- Brain Imaging Report from [11C]MePPEP in Humans
- MePPEP: Extended Acute Toxicity Study in Rats
- MePPEP: Two-week Repeat Dose Toxicity Study in Rats
- MePPEP: S. typhimurium-E. coli/Mammalian-Microsome Reverse Mutation Assay
- MePPEP: In Vitro Microwell Micronucleus Assay in CHO Cells
This application is being submitted under the exploratory IND Guidance. We wish to determine whether [18F]FMPEP-d2 is a good PET probe for the cannabinoid CB1 receptor. Our imaging and metabolism studies in monkeys suggest it will be a useful radiotracer.
We request permission for a single injection in no more than 30 healthy subjects. The injected activity will be <= 5 mCi, with an associated mass <= 10 µg. For a 70 kg subject, this corresponds to a maximum mass dose of 0.14 µg/kg. Our previous use of [18F]FMPEP-d2 in monkey brain imaging used 7.22 mCi had a corresponding mass dose of about 0.957 µg/kg.
Stanford Research Institute has performed several toxicity studies on MePPEP, a closely related analogue, and found no biologically or toxicologically significant adverse effects. See Sections IV.C through IV.F.
To date, only two CB1 PET radioligands for human use have been reported in the literature (Burns et al 2007; Yasuno et al In press). The relative poverty of good ligands for the CB1 receptor is due to the difficulty in creating a compound with the required high lipophilicity and low nonspecific binding. We have developed one of these compounds, [11C]MePPEP, and have moved it into human studies. [11C]MePPEP has proven to be a good PET tracer, however it suffers from two problems.
First, [11C]MePPEP demonstrates very low plasma concentrations shortly after peak uptake (see figure 1). Accurate measurements of plasma concentration are necessary for quantitative analysis, and very low concentrations are difficult to measure. Within 10 minutes after injection, [11C]MePPEP decreases in the plasma to about 5% of the peak achieved at 1 minute. At later time points this problem is compounded by the radioactive decay of carbon-11 (half-life = 20 minutes). By 120 minutes, only 1.6% of the original radioactivity would remain. Such lows levels of radioactive concentration challenge the sensitivity of our analytical detectors, demonstrating the limitation of carbon-11.
![Radioactivity decay corrected plasma levels of unchanged [<sup>11</sup>C]MePPEP in a human](../images/FMPEP-d2.1.jpg)
Fig. 1. Radioactivity decay corrected plasma levels of unchanged [11C]MePPEP in a human
To remedy this problem, we developed a closely related analogue to [11C]MePPEP, [18F]FMPEP (see figure 2; 18F half-life = 109 minutes). A fluorine-18 analogue permitted us to measure plasma concentrations of the radioligand more accurately at later time points. Unfortunately, early monkey studies have shown this compound to undergo metabolic defluorination, causing the fluorine-18 to be taken up in bone and disrupting the quantitative analysis of the brain. For this reason we have created [18F]FMPEP-d2, which undergoes less demethylation and defluorination due to the isotopic substitution. Deuterium substitution for hydrogen has been shown to decreased defluoromethylation before (Schou et al 2004), and does not increase the toxicity or radioactive profile, since deuterium is not a radioactive species. Furthermore, it has been our experience that nonhuman primate metabolism does not always predict radioligand metabolism in humans. The NK1 tracer SPA-RQ showed defluorination and bone uptake of fluoride during initial monkey studies; however later studies in human subjects did not show defluorination (Sprague et al 2007). Therefore, we propose moving [18F]FMPEP-d2 to human use since monkey studies may not be a reliable indicator for metabolism via defluorination.
![Structures of [<sup>11</sup>C]MePPEP, [<sup>18</sup>F]FMPEP, and [<sup>18</sup>F]FMPEP-d2](../images/FMPEP-d2Structures.png)
Fig. 2. Structures of [11C]MePPEP, [18F]FMPEP, and [18F]FMPEP-d2
The second problem encountered with [11C]MePPEP was the slow rate of washout from the brain (see figure 3). Successful PET tracers used to study pathophysiology have
a significant washout from the brain that allows for estimates of distribution volume, which is proportional to receptor density. The other CB1 PET radioligand examined
in humans, MK-9470, did not have any observable washout from the brain over a period of six hours (Burns et al 2007). The authors did not report any quantitative modeling.
Since [11C]MePPEP had an observable, albeit slow, washout, we decided to proceed with [18F]FMPEP-d2. By using an isotope with a longer half-life, [18F]FMPEP-d2
can extend the observation time of the brain washout; thus [18F]FMPEP-d2 may prove to be a superior PET radioligand for CB1 receptors.
![Time-activity curve of [<sup>11</sup>C]MePPEP in a human](../images/FMPEP-d2TimeAfterInjection.png)
Fig. 3.Time-activity curve of [11C]MePPEP in a human
![]()
The objective of this study is to characterize the pharmacokinetics of the brain uptake of [18F]FMPEP-d2 in healthy subjects by performing compartmental analysis with an arterial input function. We wish to know if time-independent values of brain distribution volume (i.e., a measure of receptor density) can be obtained in human subjects, as we have found for rhesus monkey (Section V.A). If [18F]FMPEP-d2 is amenable to quantitation in human subjects, then it may be a useful ligand to study potential pathophysiological changes of this receptor. We understand that the current exploratory IND application is for a limited number of healthy subjects. We will not proceed to any other studies without review and approval of such a plan by the FDA.
The complete protocol is located in Appendices X.B. A brief summary is included below.
Objective
The central cannabinoid receptor (CB1) is one of the most abundant neuromodulatory receptors in the brain. It is found on glutamatergic, dopaminergic and GABA-ergic synaptic terminals and belongs to G-protein coupled receptor family. The CB1 is a target for drug therapy, including the use of an antagonist as an appetite suppressant. The central cannabinoid receptor CB1 has never been visualized in humans. In collaboration with Eli Lilly, we developed a promising PET ligand for the CB1 receptor: [18F]FMPEP-d2.
Study Population
In the current protocol, we wish to evaluate [18F]FMPEP-d2 in approximately 10 healthy subjects.
Design
Brain imaging studies will consist of subject evaluation followed by PET and MRI scans.
Outcome Measures
We intend to determine the kinetics of brain uptake and washout, clearance in the plasma, and the distribution volume of [18F]FMPEP-d2 calculated with compartmental modeling. Distribution volume is proportional to the density of receptors and is equal to the ratio at equilibrium of uptake in brain to the concentration of parent radiotracer in plasma.
Objective
Should the brain imaging studies prove to be successful, we will continue with whole body dosimetry studies. Preliminary dosimetry studies with [18F]FMPEP-d2 have been performed in nonhuman primates, how ever these need to be continued in humans before further investigation of this novel tracer can continue.
Study Population
In the current protocol, we wish to evaluate [18F]FMPEP-d2 in approximately 10 additional healthy subjects.
Design
The whole body dosimetry studies will consist of subject evaluation followed by a PET scan.
Outcome Measures
We intend to determine the whole body distribution of activity and thereby calculate radiation exposure to organs of the body.
Objective
Should the brain imaging and dosimetry studies prove to be successful, we will continue with test/retest brain imaging studies. Test/retest studies with [18F]FMPEP-d2 will provide evidence of reproducibility and strengthen the assurance that this radioligand can be used to assess pathology. Previous investigations in developing a CB1 receptor PET tracer have demonstrated the need to test reproducibility (Terry et al In Writing; Burns et al 2007).
Study Population
In the current protocol, we wish to evaluate [18F]FMPEP-d2 in approximately 10 additional healthy subjects.
Design
The test/retest brain studies will consist of subject evaluation followed by PET and MRI scans.
Outcome Measures
We intend to determine the reproducibility of the outcome measures from the brain imaging, namely, distribution volume.
The CMC Section is a separate portion of this application.
The pharmacological effects and side-effects of CB1 antagonists and inverse agonists are known from three large studies of rimonabant, abbreviated RIO (Rimonabant in Obesity). RIO trials in Europe (RIO-Europa) included 1,507 patients with obesity and dyslipidiemias; RIO in North America included 3,040 patients (Pi-Sunyer et al 2006); and RIO-Lipids included 1,006 patients (Despres et al 2005). Using a double-blind, placebo-controlled design, rimonabant was administered orally at doses of 5 mg/qd and 20 mg/qd taken for one (RIO-Lipids) and two (RIO-Europe and RIO-North America) years.
The pharmacological effects included decreased appetite, weight loss and alteration of lipid and glucose metabolism with normalization of HDL cholesterol, triglycerides, and insulin resistance (Bramlage et al 2006; Gelfand and Cannon 2006a). Another effect of CB1 antagonists studied in the STRATUS (Studies with Rimonabant and Tobacco Use) series of clinical trials is facilitation of smoking cessation (Cleland et al 2004; Gelfand and Cannon 2006b).
Rimonabant was well tolerated with relatively minor side effects, including nausea, diarrhea, arthralgia, headache, nasopharyngitis, influenza, dizziness, anxiety, insomnia and depression which were greater than placebo and dose related (Despres et al 2005; Pi-Sunyer et al 2006; Van Gaal et al 2005).
In summary at daily doses of 5-20 mg for up to two years, the CB1 antagonist and inverse agonist rimonabant was well tolerated with relatively minor side effects. Thus, we expect no pharmacological effects or side effects from the proposed dose of 10 μg.
|
In membranes prepared from Sf9 cells ectopically expressing the human CB1 or CB2
receptors, in vitro functional
binding activity of non-labeled MePPEP was determined using an antibody capture scintillation proximity GTPγ[35S]
binding assay and compared to rimonabant (previously called SR141716a). MePPEP inhibited functional GTPγ[35S]
binding at the human CB1 receptor with high potency (Kb = 0.574 ± 0.207 nM), compared to rimonabant (Kb = 5.96 ± 0.209 nM).
MePPEP was significantly less potent at the human CB2 receptor (Kb = 363 77.9 nM for MePPEP and Kb > 10,000 nM for rimonabant at CB2).
FMPEP inhibited functional GTPγ[35S] binding at the human CB1 receptor with high potency (Kb = 0.634 ± 0.177 nM)
and was significantly less potent at the human CB2 receptor (Kb = 669 ± 142).
Both MePPEP and FMPEP were evaluated more broadly at common protein targets. Broad screening was performed by standard radioligand binding techniques through a contract agreement with Cerep (Paris, France). For MePPEP, competitive binding was either not significant or had a Ki value of > 10 μM at the following receptor, ion channel, or enzyme targets: acetylcholinesterase, CCK1, CCK2, EDG-2, Cox1, Cox2, adenosine A1, adenosine A3, adrenergic alpha1, adrenergic alpha2, angiotensin AT1, adrenergic beta1, adrenergic beta2, benzodiazepine site, CA2+ channel (verapamil site), dopamine D1, do-pamine D2S, GABA, Ghrelin, histamine H1, histamine H2, K+ channel (HERG), non-selective muscarinic, NE transporter, NK1, NK2, non-selective opiate , non-selective 5HT. FMPEP inhibited binding to the CA2+ channel L-diltiazem site (58.6% at 10 μM and 28.4% at 1 μM), but did not have significant activity at any other receptor listed above. |
The objective of this study is to determine potential toxic effects, and to identify potential target organs of toxicity for the toxicity endpoints examined following a single in-travenous bolus administration of C1.
Male and female Sprague-Dawley rats (10/sex/group) were given a single i.v. dose of C1 at 88.1 μg/kg (528.6 μg/mg, 100x human equivalent dose (HED)) on Day 1. A control group (10/sex) was given a single iv dose of vehicle (sterile phosphate buffered saline) at an equivalent volume on Day 1. Animals were sacrificed on Days 3 and 15 (interim and terminal necropsy, respectively).
The following parameters were evaluated: mortality/morbidity, clinical observations, body weights, food consumption, clinical pathology (hematology and serum chemistry), organ weights, necropsy macroscopic observation and microscopic histopathology.
All animals survived until their scheduled necropsy. No drug-related effects were observed for clinical observations, body weights, food consumption, clinical pathology, organ weights, macroscopic or histopathologic evaluations. Trivial changes in kidney organ weight did not correlate with histopathologic changes in the kidney. Likewise, minor, sporadic changes in clinical pathology parameters did not correlate with histopathologic or other toxicologic parameters, and are considered to be of minimal toxicologic significance.
In conclusion, a single intravenous administration of C1 to male and female Sprague-Dawley rats at 88.1 μg/kg (100x human dose) did not produce overt biologically or toxicologically significant adverse effects. The maximum tolerated dose (MTD) is therefore considered to be greater than 88.1 μg/kg (528.6 μg/m2) and the no observed adverse effect level (NOAEL) is considered to be at least 88.1 μg/kg (528.6 μg/m2).
|
The purpose of this study was to evaluate the toxicity
of Compound 2463608 when administered daily by intravenous injection to rats for at least 2 weeks.
The toxicity of Compound 2463608 was evaluated in male and female Crl:CD(SD) rats (five/sex/group) given saline control [0.9% Sodium Chloride for Injection, USP (sterile saline)], vehicle control [20% (w/v) Captisol in 25mM acetate buffer prepared in Sterile Water for Injection, USP, pH 3.8 to 4.4], or 1.0 mg of Compound 2463608/kg of body weight (mg/kg) via slow bolus intravenous injection daily for 15 days. All animals survived to scheduled sacrifice. The only compound-related clinical sign seen during the dosing phase was excessive grooming. No compound-related changes in ophthalmic examination findings, body weight, or food consumption were noted. No compound-related effects were seen on clinical pathology test results, organ weights, or microscopic morphology. All findings were attributed to vehicle or injection procedure. Males given the vehicle control or 1.0 mg/kg had statistically significantly decreased mean absolute thymus weight, mean thymus-to-body weight percentage, and mean thymus-to-brain weight percentage when compared with the saline control group, and females given 1.0 mg/kg had statistically significant increases in these same thymus weight parameters when compared with females given the vehicle control. No correlative macroscopic or microscopic thymus findings were seen in either sex. Decreased thymus weight in males in the groups given vehicle only or Compound 2463608 and in females given vehicle only were attributed to the vehicle. Increases in thymus weight in females given Compound 2463608 were considered spurious since 4/5 animals had thymus weights within the range of concurrent saline controls. Minimal to moderate vacuolation of tubule cells in the kidney was a microscopic finding seen in all animals given either the vehicle control or the test compound. No animals given the saline control were similarly affected, indicating that the tubular vacuolation was associated with the vehicle control article. The incidence and severity of microscopic findings at injection sites were similar across all groups, suggesting that they were due to the injection procedure and not to the vehicle or Compound 2463608. In summary, daily intravenous administration of 1.0 mg Compound 2463608/kg to Crl:CD(SD) rats for 15 days resulted in the compound-related clinical sign of excessive grooming but no adverse findings. The no observed adverse effect level is, therefore, 1.0 mg/kg under the conditions of this study. |
|
The objective of this study was to evaluate 2463608 (Test Article: 2463608) and/or its metabolites for the ability to induce reverse mutations at the histidine locus in Salmonella typhimurium tester strains TA98, TA100, TA1535, and TA1537, and at the tryptophan locus of Escherichia coli tester strain WP2uvrA. Evaluations were conducted in
the presence or absence of an exogenous mammalian activation system (S9) containing microsomal enzymes.
The preliminary rangefinding mutagenicity assay was performed using all tester strains at 1.60, 5.00, 16.0, 50.0, 160, 500, 1600, and 5000 mcg of 2463608 per plate along with the appropriate vehicle and positive controls. The preliminary rangefinding mutagenicity assay was performed in the presence and absence of S9 mix. All doses of test article, vehicle controls and positive controls were plated in duplicate. The confirmatory mutagenicity assay was performed using all tester strains in both the presence and absence of S9 mix at 33.3, 100, 333, 1000, 2000, and 5000 mcg of 2463608 per plate. All doses of test article, vehicle controls and positive controls were plated in triplicate. The results of the Salmonella typhimurium-Escherichia coli/Mammalian-Microsome Reverse Mutation Assay with a Confirmatory Assay indicate that, under the conditions of this study, 2463608 (Test Article: 2463608) did not cause a positive increase in the mean number of revertants per plate with any of the tester strains either in the presence or absence of microsomal enzymes prepared from AroclorTM-induced rat liver (S9). The responses to the positive controls demonstrated that the test system was sensitive to chemical mutagens. |
|
The objective of this study was to assess the capability of the test article, 2463608, to induce clastogenicity/aneugenicity
in Chinese hamster ovary cells (CHO-WBL) by measuring the extent of micronucleus formation.
In the dose range-finding assay, the CHO-WBL cells were treated for ~4 hours in the presence and absence of S9 metabolic activation and ~23 hours in the absence of metabolic activation, and cultures were harvested ~24 hours after initiation of treatment. Cytotoxicity was assessed by the appearance of the cultures in each well and by cell cycle kinetics. Doses tested in the micronucleus assay were selected based on the results of a dose range finding assay. In the micronucleus assay, the CHO-WBL cells were treated for ~4 hours in the presence and absence of S9 metabolic activation and ~23 hours in the absence of metabolic activation, and cultures were harvested ~24 hours after initiation of treatment. The doses selected for evaluation were 75.0, 150, 225, and 550 mcg/mL in the ~4-hour trial in the presence of S9 metabolic activation, 75.0, 150, 225, and 300 mcg/mL in the ~4-hour trial in the absence of S9 metabolic activation, and 12.5, 37.5, and 75.0 mcg/ml in the ~23-hour trial in the absence of S9 metabolic activation. Statistical significance was observed at test article concentrations of 150 mcg/mL in the ~4-hour exposure period in the presence of metabolic activation and 225 mcg/mL in the ~4-hour exposure period in the absence of metabolic activation. However, these responses were not dose dependent and were within the historical control range for the vehicle controls. Therefore, these responses are not biologically relevant. The test article, 2463608, was considered negative for inducing micronuclei under the conditions of the assay. |
[18F]FMPEP-d2 has never been administered to human subjects. However, [11C]MePPEP has been administered to 14 human subjects at tracer doses (i.e., < 10 μg) with no serious adverse events.
Under the exploratory IND of [11C]MePPEP we studied two groups, each with 7 healthy subjects. The first group received whole body imaging to estimate radiation exposure to organs of the body. The Effective Dose was 28 mrem/mCi. The second group of subjects had serial brain imaging and serial sampling of arterial blood to measure the concentration of [11C]MePPEP separated from radiolabeled metabolites. The internal report of our results is included in Appendix X.G. The radioligand was safe and well tolerated by all 14 subjects. No adverse effects could be linked to the radioligand. See page 7 of 15 in the report (Appendix X.G).
We have completed initial evaluations of [18F]FMPEP-d2 in nonhuman primates (see figure 4). As expected, [18F]FMPEP-d2 behaved very similar to [11C]MePPEP. The longer scan time permitted by fluorine-18 allowed a greater observation of the radioligand washout from the brain.
![Time-activities curve of [<sup>18</sup>F]FMPEP-<em>d</em><sub>2</sub> in monkey](../images/FMPEP-d2TimeAfterInjectionMonkey.png)
Fig. 4. Time-activities curve of [18F]FMPEP-d2 in monkey. Solid symbols are from a baseline study; open figures are from a study after pretreatment with 3 mg/kg of rimonabant
Our PET studies with [11C]MePPEP in nonhuman primates is described in Neuropsycho-pharmacology (Yasuno et al In press). The complete paper is located in Appendix F, and the abstract is copied below.
|
The cannabinoid CB1 receptor is one of the most abundant G protein-coupled receptors in brain and is a promising target of therapeutic drug development. Success of drug development for neuropsychiatric indications is significantly enhanced with the ability to directly measure spatial and temporal binding of compounds to receptors in central compartments. We assessed the utility of a new PET (positron emission tomography) radioligand to image CB1 receptors in monkey brain. [11C]MePPEP ((3R,5R)-5-(3-methoxy-phenyl)-3-((R)-1-phenyl-ethylamino)-1-(4-trifluoromethyl-phenyl)-pyrrolidin-2-one) has high
CB1 affinity (Kb = 0.574 0.207 nM) but also moderately high lipophilicity (measured LogD7.4 = 4.8).
After intravenous injection of [11C]MePPEP, brain activity reached high levels of almost 600% SUV (standardized uptake value) within 10-20 min. The regional uptake was consistent with the distribution of CB1 receptors, with highest radioactivity in cerebellum and striatum and lowest in thalamus and pons. Injection of pharmacological doses of CB1-selective agents confirmed that the tracer doses of [11C]MePPEP reversibly labeled CB1 receptors. Preblockade or displacement with two CB1 selective agents (ISPB (4-(3-cyclopentyl-indole-1-sulfonyl)-N-(tetrahydro-pyran-4-ylmethyl)-benzamide) and rimonabant) showed that the majority (> 85%) of brain uptake was specific and reversibly bound to CB1 receptors. [11C]MePPEP was rapidly removed from arterial plasma. Regional brain uptake could be quantified as distribution volume relative to the concentration of parent radiotracer in plasma. Approximately 90 min of scanning provided relatively stable measurements of distribution volume, consistent with little, if any, radiometabolites entering brain. The P-glycoprotein (P-gp)inhibitor DCPQ ((R)-4-[(1a,6,10b)-1,1-dichloro-1,1a,6,10b-tetrahydrodibenzo[a,e]cyclopropa[c]cyclohepten-6-yl]- [(5-quinolinyloxy)methyl]-1-piperazineethanol) did not significantly increase brain uptake of [11C]MePPEP, suggesting it is not a substrate for this efflux transporter at the blood-brain barrier. [11C]MePPEP is a radioligand with high brain uptake, high specific signal to CB1 receptors, and adequately fast washout from brain that allows quantification with 11C (half-life = 20 min). These promising results in monkey justify studying this radioligand in human subjects. |
We previously described important pharmacological parameters of MePPEP in our PET im-aging paper with regard to clearance and effects. The relevant paragraphs of the paper Yasuno et al., (In press) are reproduced below.
We observed similar results in our studies of [18F]FMPEP-d2. The radioligand was quickly metabolized and represented 89%, 39%, 19%, 6%, and 3% of total plasma activity at 2, 5, 10, 30, and 60 min, respectively. [18F]FMPEP-d2 was rapidly removed from arterial plasma, with a clearance rate of 512 mL min-1. Plasma activity of unchanged [18F]FMPEP-d2 peaked at ~1 min and decreased to 2.5% of the peak by 10 min.We also performed a blocking study using rimonabant (3.0 mg/kg) and observed the same results reported above (EFFECTS).
The peak uptake of [18F]FMPEP-d2 in monkey cerebellum and striatum was approximately 600% SUV (standardized uptake value), where SUV is calculated as
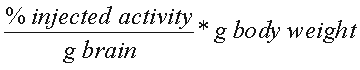 .
We and others have used SUV to make comparison between species - e.g., monkey to man. For the proposed injection of no more than 10 g, this corresponds to a concentration of 1.90 nM. This is calculated as follows:
.
We and others have used SUV to make comparison between species - e.g., monkey to man. For the proposed injection of no more than 10 g, this corresponds to a concentration of 1.90 nM. This is calculated as follows:
Assumptions: body weight = 70,000 g SUV = 600% molecular weight = 461
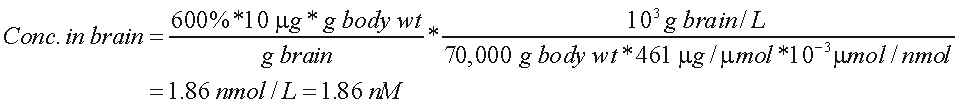
Thus, at peak uptake, [18F]FMPEP-d2 would be 1.86 nM. The density of CB1 receptors throughout the mouse brain is 1810 nM/mg protein (Abood et al 1997). Assuming 10% of the brain weight is protein, this density corresponds to 181 nM. If all tracer were bound to receptor (which is not true because of nonspecific uptake), then the occupancy would be 1% (1.9 nM / 181 nM).
[18F]FMPEP-d2 has never been administered to humans. We have administered a closely related analogue, [11C]MePPEP, to 14 subjects with no significant adverse events. Our experience of [11C]MePPEP as a brain imaging agent for CB1 receptors is summarized in a report (see Appendix X.G). In brief, [11C]MePPEP demonstrated promising qualities of a PET tracer for human use, however it is limited by a slow washout from the brain and a difficulty in measuring plasma levels at late time points. Both of these limitations may be resolved by using a ligand with a longer radioactive half-life, such as [18F]FMPEP-d2. A longer scanning time would allow for a longer observation of brain washout, and a greater radioactivity at later time points would allow more accurate plasma measurements.
Radiation dosimetry for [18F]FMPEP-d2 in humans has been estimated from studies in nonhuman primates (Appendix X.E.). The Effective Dose is 78 mrem/mCi.
A categorical exclusion is requested regarding the environmental assessment. Activity excreted by the subjects is disposed through the sewage system at such low amounts that there will be no detrimental effects to the environment.
All potentially contaminated laboratory materials are held at least 10 half lives for decay. In addition, these materials are surveyed to ensure background levels of activity prior to disposal through regular waste systems.
The CRF is in FMPEP-d2AppendixX.D.
